Correct Laser Wavelength for Raman Material Identification
Sponsored by B&W TekJan 25 2021
Over the years, dispersive Raman spectroscopy has been increasingly employed for sample analysis in the areas of biomedical research, material identification, archeology and art, and forensics thanks to its sampling flexibility and portability.
When selecting a Raman instrument, one of the main requirements is the wavelength of the laser that is built into the Raman spectrometer system.
The specific peak position and Raman signature of any material relates to its individual chemical structure and are not affected by the excitation wavelength, so the molecular fingerprint will remain consistent despite the excitation laser wavelength.
Despite this, the user’s selection of Raman excitation wavelength can enhance the measurement of various samples because different excitation wavelengths offer particular advantages and disadvantages.
This leads to the question of how a laser excitation wavelength can be chosen for the application in question. There are a number of different excitation choices, but 532 nm, 785 nm and 1064 nm are the three that are most commonly used.
The 785 nm excitation system is the most widespread as it provides the ideal balance of sensitivity to fluorescence, cost, signal strength and total performance and can be employed to gather the Raman spectrum of many organic materials in an efficient manner.
The longer wavelength of 1064 nm is advantageous when samples are fluorescent in the other wavelengths.
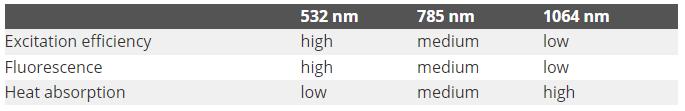
The table below outlines several key performance indicators for these three wavelengths
Source: B&W Tek
The excitation efficiency is the most apparent difference. Where λ is the wavelength of the laser, Raman scattering efficiency is proportional to λ-4.
As an example, Raman scattering at 532 nm when compared to 785 nm is a factor of 4.7 more efficient and is 16 times faster than at 1064 nm. This means that when using longer wavelengths, the scan time is much longer compared to the scan time taken to acquire the spectrum when using 532 nm, on the basis that all other parameters are consistent.

For 785 nm systems, the Raman signals are distributed in the NIR range (750 to 1050 nm), where the response is still comparatively good. For 1064 nm, NIR-sensitive InGaAs array detectors are usually employed for dispersive instruments because there is no response from silicon above 1100 nm.
Due to concerns with cost control, the majority of dispersive 1064 nm Raman instruments are supplied with a 512-pixel sensor (compared to 2048 for many others). This results in a comparatively low pixel resolution in the detector and a potentially smaller coverage of Raman shift.
Fluorescence is another key phenomenon that arises and interrupts the measurement of the Raman spectrum. In the majority of applications where excitation efficiency is crucial, this is a decisive factor.
While Raman scattering and fluorescence are created through highly similar processes, fluorescence is produced by a photoluminescence mechanism. Consistent separation from the excitation frequency is achieved by the Raman peaks, whereas fluorescence stays at a particular wavelength or frequency, which means that it does not change with the excitation laser.
Fluorescence signals also decline over time due to the fluorescence bleaching effect. Laser excitation at longer wavelengths is preferred to limit fluorescence interference in a Raman spectrum. Fluorescence may be more prevalent when analyzing dyes, natural products and darker samples.
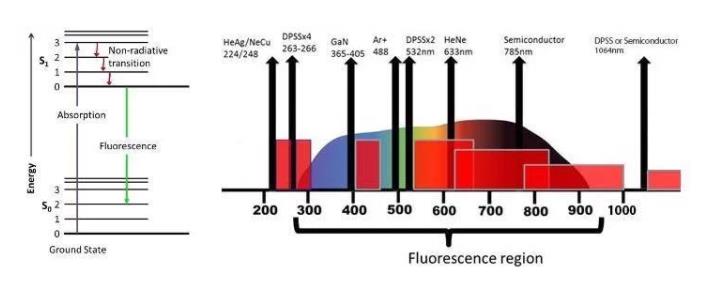
Image Credit: B&W Tek
Laser energy absorption by samples should additionally be considered as this can lead to changes in the sample and can result in sample heating. In general, longer excitation wavelengths cause the sample to absorb more light and cause more sample heating.
Small volume liquid samples can be boiled in extreme cases, while black, colored, or dark samples can be damaged. Sample damage as a result of laser energy absorption can be reduced or avoided through sample rotation or by decreasing the laser power density at the sample, but these procedures increase the measurement time and/or add complexity to the process.
With inaccurate measurement configurations, it is possible for sample damage to arise because of improper handling, even though Raman is a non-destructive technology. Additional factors, like the resonance Raman effect, should additionally be considered when selecting a wavelength.
The image below displays sample spectra that shows the varying performance of different excitations. It is important to note that there is a range of materials that can be analyzed with the use of any excitation wavelength with no issues.
The example below demonstrates that the Raman spectrum of toluene can be easily acquired through the use of all three standard excitation lasers.
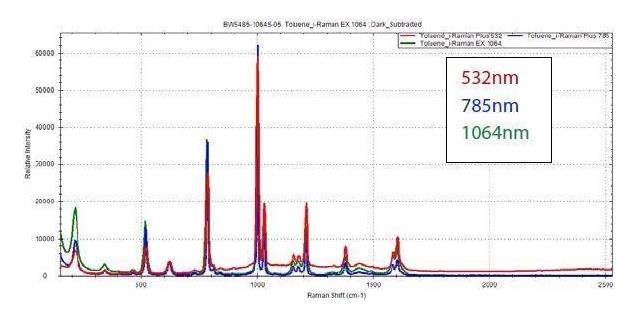
Image Credit: B&W Tek
The 532 nm laser excitation offers high sensitivity and is most often used for the analysis of carbon nanotubes, where the sample may burn at 785 nm.
There is the option to decrease the laser power for the higher wavelength, but this will produce a lower SNR. The 532 nm excitation is also suggested for metal oxides or inorganic materials and minerals more generally.
Another advantage of the 532 nm instrument is that it covers the entire range from 65 cm-1 to 4000 cm-1. This may be a crucial consideration for specific applications where there are clear signals in the higher Raman shift region, such as the –NH and –OH functional groups in the range of 2800 and 3700 cm-1.
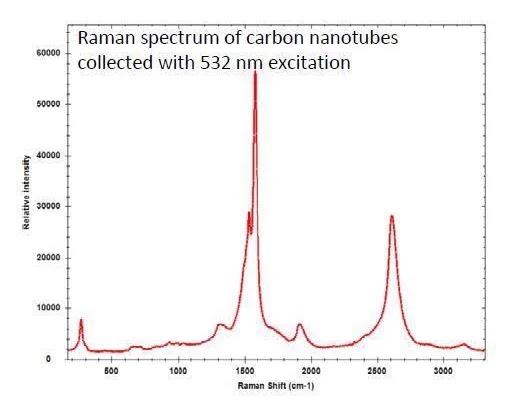
Image Credit: B&W Tek
The most widespread and popular wavelength used is the 785 nm wavelength excitation because it performs effectively for more than 90% of Raman active materials with reduced fluorescence interference.
One scan acquisition can take anywhere from one second to a few minutes according to the sample and the strength of the related Raman signal. Out of the three standard wavelengths, the 785 nm is the most common choice due to its balance between spectral resolution and fluorescence reduction.
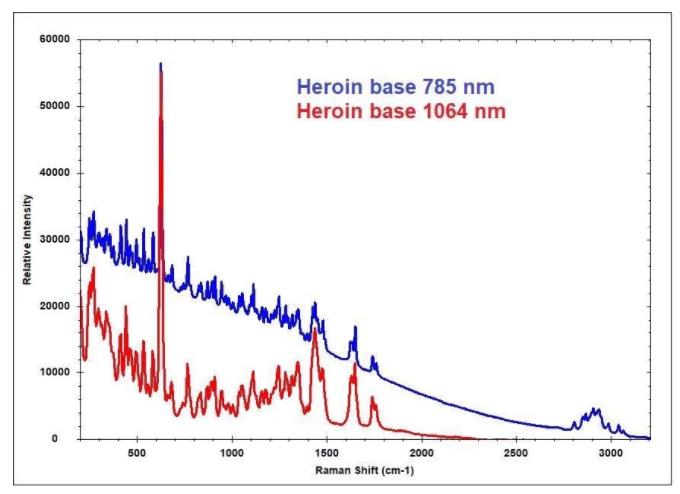
Image Credit: B&W Tek
785 nm and 1064 nm excitation were used to scan the spectra to the left of the heroin base. Due to having a higher resolution, the 785 nm spectrum exhibits greater detail but does have a sloping baseline caused by fluorescence.
It was also collected over a much faster integration time compared to the 1064 nm, lasting 10 seconds versus high tens of seconds. 1064 nm laser excitation is selected in most applications to reduce fluorescence.
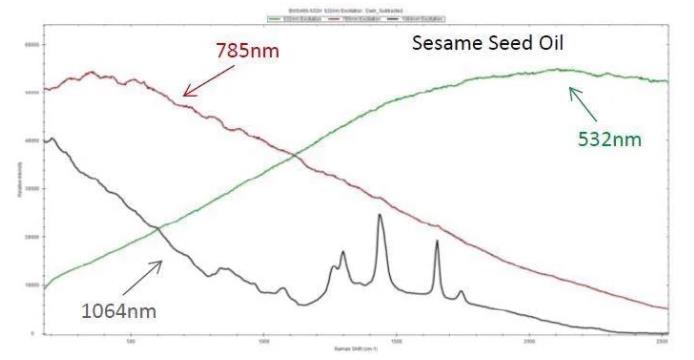
Image Credit: B&W Tek
As an example, 1064 nm excitation can be used to measure the Raman spectrum of sesame seed oil, which is a dark liquid, whereas strong fluorescence covers the Raman features in the spectra acquired from 785 nm and 532 nm.
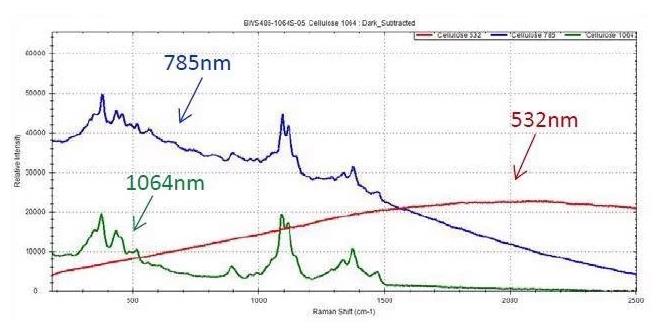
Image Credit: B&W Tek
While fluorescence in cellulose has been a topic of concern in the past, 1064 nm and 785 nm can be used to acquire a good spectrum, with the former offering a lower background contribution. Fluorescence is an issue when quantifying the Raman spectrum of cellulose only when measured utilizing 532 nm.
To summarize:
- The 532 nm laser delivers the greatest energy to bombard the structure of the sample, which results in more fluorescence, making it most suitable for inorganic materials.
- The 785 nm laser offers a balance of performance, reduced fluorescence and less excitation efficiency, providing the best cost performance and making it the ideal selection for the majority of chemicals.
- The 1064 nm laser is least impacted by fluorescence but also introduces a higher risk of sample heating if no special care is taken and takes a comparatively longer acquisition time to get sufficient levels of signal to measure. This means that it is most suitable for darker and colored materials like colored polymers, oils, dyes and natural products.
This information has been sourced, reviewed and adapted from materials provided by B&W Tek.
For more information on this source, please visit B&W Tek.







