Effectiveness of Six Irrigation Techniques With Sodium Hypochlorite in Tissue Dissolution
He Liu • Ya Shen • Markus Haapasalo
Published: May 18, 2023 DOI: 10.7759/cureus.39208
Cite this article as: Liu H, Shen Y, Haapasalo M (May 18, 2023) Effectiveness of Six Irrigation Techniques With Sodium Hypochlorite in Tissue Dissolution. Cureus 15(5): e39208. doi:10.7759/cureus.39208
Abstract
Introduction: Complete removal of pulp tissue by mechanical instrumentation remains a challenge. The study aimed to assess the efficacy of six irrigation techniques using 3% sodium hypochlorite (NaOCl) in dissolving tissue.
Materials and methods: Seventy standardized fragments of bovine muscle tissue were divided into seven groups and exposed to different irrigation devices, with a maximum of 10 minutes of irrigation at room temperature. The devices included erbium-doped yttrium-aluminum-garnet (Er:YAG) laser (LightWalker® ST-E; Fotona d.o.o., Ljubljana, Slovenia), erbium, chromium-doped yttrium, scandium, gallium and garnet (Er,Cr:YSGG) laser A (EdgePRO™; Biolase Technology Inc., San Clemente, California, United States), Er,Cr:YSGG laser B (WaterLase iPlus®; Biolase, California, United States), multisonic ultracleaning system (MUS) GentleWave® system with CleanFlow™ handpiece (Sonendo, Inc., Laguna Hills, California, United States), passive ultrasonic irrigation (PUI), EndoUltra ultrasonic activator (Vista Apex Dental Products, Racine, Wisconsin, United States), and conventional needle irrigation (CNI), a 29G NaviTip irrigation needle at a flow rate of 5 mL/min (Ultradent Products, Inc., Utah, United States), with 3% NaOCl without agitation as the control. For samples that dissolved completely within 10 minutes, the dissolution time was recorded, and for other samples, the tissue weight was measured after the 10-minute irrigation experiment. The tissue dissolution rate (%/s) was calculated for all experiments.
Results: MUS had the fastest tissue dissolution rate (P < 0.01), at 2.986% ± 0.395% per second. The laser systems had dissolution rates of 0.388% ± 0.062% per second for Er:YAG laser, 0.316% ± 0.042% for Er,Cr:YSGG laser A, and 0.258% ± 0.018% for Er,Cr:YSGG laser B. CNI and PUI showed the slowest tissue dissolution rates.
Conclusions: MUS with CleanFlow, followed by Er:YAG and Er,Cr:YSGG lasers, achieved a significantly faster tissue dissolution rate than conventional irrigation methods of PUI and CNI tested. MUS with CleanFlow can greatly improve the effectiveness of NaOCl in tissue dissolution.
Introduction
Complete removal of pulp tissue in endodontic treatment remains challenging due to the complex anatomies and irregular morphology of the root canal system [1,2]. Root canal irrigation is crucial for removing tissue remnants left by mechanical debridement [3,4]. Sodium hypochlorite (NaOCl) is considered the first choice as an irrigant solution in endodontic treatment because it is the only solution used in endodontics that can dissolve organic matter, such as necrotic pulp tissue and root canal biofilm, both of which are crucial for the success of the treatment [5,6]. It also has excellent antimicrobial activity. Furthermore, NaOCl is a relatively low-cost and readily available solution. It is easy to use, and the irrigant can be delivered through a variety of irrigation devices, such as syringes, needles, and ultrasonic instruments. Previous studies have indicated that laser-activated irrigation techniques, such as erbium-doped yttrium aluminum garnet (Er:YAG) and erbium, chromium, yttrium, scandium, gallium garnet (Er,Cr:YSGG) lasers, can enhance the effectiveness of NaOCl in tissue dissolution [7,8]. A new Er,Cr:YSGG laser system, EdgePRO™ (Biolase Technology Inc., San Clemente, California, United States), has been introduced recently for cleaning, debridement, and disinfection of the root canal system. However, there is no published study on the effectiveness of EdgePRO with NaOCl in tissue dissolution.
A multisonic ultracleaning system (MUS), GentleWave® (Sonendo Inc, Laguna Hills, California, United States) has been developed for cleaning the root canal system. It generates a broad spectrum of soundwaves and, according to some studies, can create advanced fluid dynamics [9-11]. A previous study has shown that MUS using a molar handpiece with NaOCl dissolves soft tissue much faster than conventional irrigation methods using syringe-needle irrigation or ultrasound [9]. Recently, a new handpiece called CleanFlow™ ((Sonendo, Inc.) was introduced in MUS. Unlike previous handpiece designs, no part of the CleanFlow handpiece is placed in the access cavity or pulp chamber, and the mechanism by which fluid dynamics is created is also different. However, there is no data on the soft tissue-dissolving capacity of MUS with the CleanFlow handpiece. Moreover, there are no in vitro or in vivo studies that have compared lasers and MUS.
Therefore, the aim of this study was to quantitatively evaluate and compare the in vitro soft tissue-dissolving effectiveness of Er:YAG laser, Er,Cr:YSGG laser, MUS with the CleanFlow handpiece, and two conventional root canal irrigation methods. The hypothesis tested was that there was significant difference in the effectiveness of irrigation activation techniques tested with 3% NaOCl in tissue dissolution.
Materials & Methods
Devices used for irrigation
The endodontic devices and instruments used in the experiment included the following: (i) LightWalker® ST-E laser system (Fotona d.o.o., Ljubljana, Slovenia), (ii) EdgePRO laser system, (iii) WaterLase iPlus® laser system (Biolase Technology Inc.), (iv) MUS GentleWave® system with CleanFlow™ handpiece, (v) passive ultrasonic activation (PUI) with a cordless ultrasonic activator (EndoUltra; Vista Apex Dental Products, Racine, Wisconsin, United States) with a NiTi activation tip (size 20, 0.02 taper), and (vi) conventional needle irrigation (CNI) with a 29G open-ended syringe needle (NaviTip; Ultradent Products, Inc., South Jordan, Utah, United States). The control group consisted of 3% NaOCl without any agitation.
Tissue specimen preparation
Seventy pieces of bovine muscle tissue were used in this study. The same fresh bovine meat was used to prepare the tissue specimens, which were then stored frozen at -20°C and left to thaw at room temperature before being cut into equal-sized pieces measuring 2 x 2 x 1 mm using a stainless-steel blade (Cincinnati Surgical Company Inc., Cincinnati, Ohio, United States). After blotting dry with a paper towel, the tissue specimen mass was measured using a calibrated electronic balance (FX-300; A&D Company Ltd, Toshima City, Tokyo, Japan) to ensure that each tissue sample had an initial mass of 10 ± 0.5 mg. The tissue samples were then divided into seven groups (n=10 each): Er:YAG laser (LightWalker ST-E), Er,Cr:YSGG laser A (EdgePRO), Er,Cr:YSGG laser B (WaterLase iPlus), MUS, PUI (EndoUltra) with a NiTi activation tip (size 20, 0.02 taper), conventional needle irrigation (CNI) using a positive-pressure 29G NaviTip irrigation needle at a flow rate of 5 mL/min, and a control group (no agitation). There were no significant differences in the tissue sample mass used for each group (P > 0.05).
Tissue dissolution
To deliver the irrigant to the experimental apparatus, a 60 mL syringe filled with 3% NaOCl was connected to a 29G NaviTip irrigation needle using a Luer lock connection to Tygon ST tubing (Ismatec, Wertheim, Germany). A digitally controlled syringe pump (Fusion 200; Chemyx Inc., Stafford, Texas, United States) was used to ensure a precise flow rate of 5 mL/min. Before each use, the syringe pump and needle flow rate were calibrated as previously described [9]. To prevent the tissue specimen in the lower portion of the glass tube from being flushed out during the agitation process, a thin nylon mesh was inserted inside the tube, 10 mm from the top end of the glass tube, to mimic a pulp chamber and provide space for the different technologies to work. A thin glass tube was set below the nylon mesh to help stabilize the tissue specimen in the center.
Figure 1 illustrates the experimental setup for measuring tissue dissolution. The volume of the upper and lower tubes was 0.28 mL and 0.14 mL, respectively. Furthermore, to ensure that the same amount of suction was provided for all devices, suction was provided by the MUS at the top of the model.
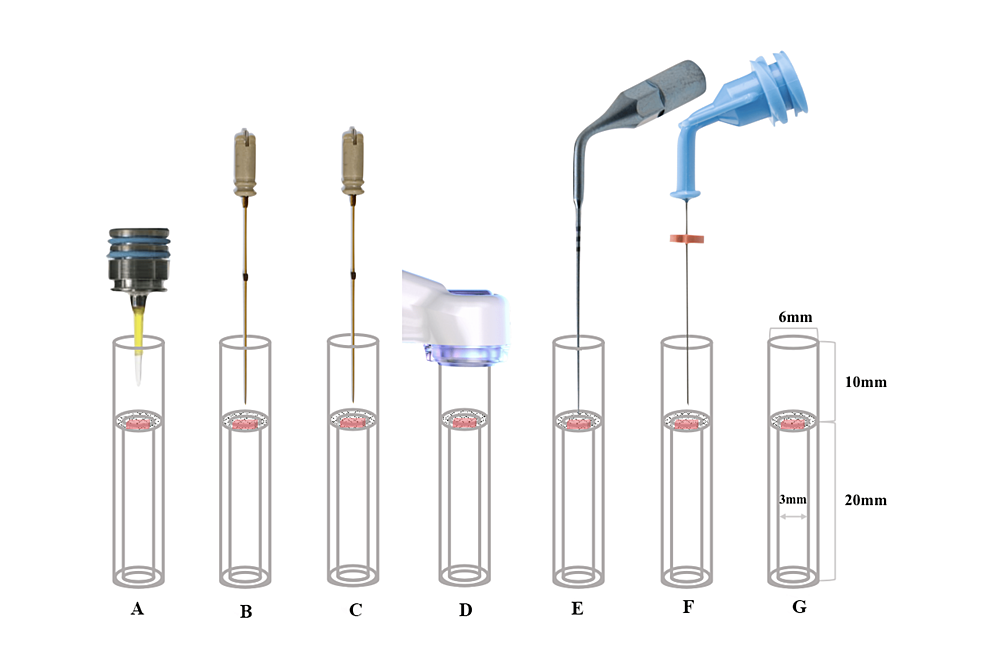
Figure 1: Schematic diagram drawing showing irrigation techniques tested in tissue dissolution. A: Er:YAG laser; B: Er,Cr:YSGG laser A; C: Er,Cr:YSGG laser B; D: MUS; E: PUI; F: CNI; G: Control
MUS: multisonic ultracleaning system; PUI: passive ultrasonic irrigation; CNI: conventional needle irrigation; Er:YAG: erbium-doped yttrium-aluminum-garnet; Er,Cr:YSGG: erbium, chromium-doped yttrium, scandium, gallium, and garnet
In the three laser groups, PUI, CNI, and control groups, the 29G NaviTip irrigation needle was placed into the upper portion of the glass vial at a depth of 3 mm along the wall and continuously delivered 3% NaOCl solution into the upper portion at 5 mL/min, as described above. In the MUS group, the 29G NaviTip irrigation needle was not used. If the tissue sample was completely dissolved visually within 10 minutes, the time (in seconds) it took to dissolve the tissue was recorded. For all other tissue samples that were not completely dissolved, the samples were blotted dry with a paper towel, and the remaining tissue mass was measured and recorded.
Er:YAG Laser
The LightWalker ST-E console was set to SSP mode at 15 Hz, 20 mJ, and 0.3W, and the air-water spray was turned off. An experimental photon-induced photoacoustic streaming (PIPS) tip, which was 9 mm long and 600 μm in diameter, was used. The tip was positioned centrally in the upper portion of the test tube, 5 mm away from the tissue sample, and continuously activated.
Er,Cr:YSGG Laser A
The EdgePRO console was set to 50 Hz, 25 mJ, and 1.25 W, with the air-water spray turned off. A ONE-25 tip was centrally placed into the upper portion of the test tube, 3 mm away from the tissue sample, and activated continuously.
Er,Cr:YSGG Laser B
The WaterLase iPlus console was set to 50 Hz, 25 mJ, and 1.25 W, and the air-water spray was turned off. A RFT2-25 tip was positioned at the center of the upper part of the test tube, 3 mm away from the tissue sample, and activated continuously.
MUS
In the experiment, a CleanFlow handpiece from the GentleWave system was used. To maintain appropriate fluid dynamics, the space between the handpiece and the upper end of the glass tube was sealed with Loctite 4311 (Henkel AG & Co., Düsseldorf, Germany) to ensure an airtight seal. The CleanFlow handpiece was connected to the GentleWave console following the manufacturer's instructions. The vacuum created was due to the mechanism of action generated by the GentleWave console, which was set and operated according to the manufacturer's recommendations. Approximately 45 mL of 3% NaOCl was delivered into the access cavity per minute.
PUI
A #20/0.2 titanium activator tip was centrally positioned in the upper part of the test tube, at a distance of 1 mm from the tissue sample and activated continuously at a frequency of 40,000 Hz.
CNI
A 29G NaviTip irrigation needle, which generated a positive pressure, was positioned at the center of the upper portion of the test tube and constantly delivered 3% NaOCl solution into the upper reservoir at a flow rate of 5 mL/min.
Control Group
The test tube was filled with 3% NaOCl solution.
Data analysis
The tissue dissolution rate was calculated as the percent tissue mass loss per second. The data were analyzed using GraphPad Prism software version 9.4.1 (Graphpad Software, San Diego, California, United States). The normality of distribution and the homogeneity of variance were determined. The data of each group were normally distributed. One-way analysis of variance followed by Tukey post-hoc pairwise comparisons for multiple comparisons were used for the intergroup comparisons of the mean tissue dissolution rates of the different irrigation techniques. For all analyses, statistical significance was set at a predetermined level of α = 0.05.
